Creatine kinase isoenzyme (CK-MB)
Creatine kinase isoenzyme (CK-MB) assay kit (magnetic microparticle chemiluminescence method) (hereinafter referred to as this kit) is used for in vitro quantitative detection of creatine kinase isoenzyme (CK-MB) content in human serum.
Keywords:
Myocardium
Category:
Myocardium
Brochure Download:

Tel:
Creatine kinase isoenzyme (CK-MB) assay kit
Instructions for use (Magnetic microparticle chemiluminescence method)
Instruction of Creatine kinase-MB (CK-MB) Detection Kit(Magnetic Solid Phase Chemiluminescent Immunoassay)
【Product Name】
Generic Name: Creatine kinase isoenzyme (CK-MB) assay kit (magnetic microparticle chemiluminescence method)
English Name: Creatine kinase-MB (CK-MB) Detection Kit (Magnetic Solid Phase Chemiluminescent Immunoassay)
【Packaging Specifications】
50 tests/box, 100 tests/box, 200 tests/box.
Calibrator: 6 × 1 bottle (optional), 2 × 1 bottle (optional)
Quality control product: 2 × 1 bottle (optional)
【Intended Use】
The creatine kinase isoenzyme (CK-MB) assay kit (magnetic microparticle chemiluminescence method) (hereinafter referred to as this kit) is used for in vitro quantitative detection of creatine kinase isoenzyme (CK-MB) content in human serum.
Used for the detection of acute myocardial infarction (AMI), it also plays an auxiliary role in the detection and prognosis assessment of other diseases. CK-MB mainly exists in the form of MB2 in cardiomyocytes. When cardiomyocytes are damaged, MB2 is released, and the serum CK-MB level increases in a short period of time. CK-MB can reflect cardiomyocyte damage earlier. When cardiomyocytes are damaged, CK-MB is released into the blood and rapidly increases within 6 h, reaches its peak at 24 h, and begins to decrease after 72 h and gradually returns to normal. CK-MB has a shorter half-life. When monitoring myocardial re-injury, it is more sensitive than troponin. CK-MB is a sensitive and specific indicator of myocardial injury and has been widely used clinically. Research results show that the sensitivity of CK-MB in myocardial injury is 97.5%, and the specificity is 100%. The increase in CK-MB is closely related to the area and degree of infarction. The higher the CK-MB level, the greater the myocardial damage and the more severe the necrosis. Therefore, the concentration of CK-MB in serum is an important indicator for the diagnosis and prognosis of AMI. Common laboratory diagnostic methods in clinical practice include immunochromatography, latex immunoturbidimetry, and enzyme-linked immunosorbent assay.
【Test Principle】
This kit uses a direct sandwich method: biotin-labeled CK-MB antibody, acridinium ester (AE)-labeled CK-MB paired antibody, and CK-MB in the sample, calibrator, or quality control product are combined to form a "sandwich" complex. Then, streptavidin-coated magnetic microparticles are added. Through the specific binding of streptavidin and biotin, the antigen-antibody complex is connected to the magnetic particles. Under the action of an external magnetic field, the complex formed by the immunoreaction is separated from other unbound substances. After washing the complex, a pre-excitation solution and an excitation solution are added. Acridinium ester forms an unstable excited intermediate under the action of the pre-excitation solution and the excitation solution. When the excited intermediate returns to the ground state, it emits photons, forming a chemiluminescent reaction, which can be used to detect the luminescence intensity of the reaction using a chemiluminescence instrument. Within the detection range, the luminescence intensity is proportional to the content of CK-MB in the sample. The concentration of CK-MB in the sample can be calculated using a four-parameter Logistic equation.
【Main Components】
1. Product Composition
CK-MB magnetic microparticle reagent: 1 bottle, streptavidin-coated magnetic microparticles;
CK-MB anti-reagent 1: 1 bottle, biotin-labeled CK-MB antibody conjugate;
CK-MB anti-reagent 2: 1 bottle, acridinium ester (AE)-labeled CK-MB antibody conjugate;
Calibrator (lyophilized powder): 6 bottles or 2 bottles (optional), containing BSA buffer with different amounts of CK-MB antigen added. The calibrator value is obtained by scanning the calibrator QR code. After dissolving at room temperature, it is detected;
Quality control product (lyophilized powder): 1 bottle each of high and low points (optional), containing BSA buffer with different amounts of CK-MB antigen added. The target range of the quality control product is obtained by scanning the quality control product QR code. After dissolving at room temperature, it is detected.
Note ①: Reagents from different batches of kits are prohibited from being used interchangeably.
Note ②: Six calibration points or two calibration points can be optionally selected for the calibrator. If the calibrator and quality control product have been provided to the user, the kit does not need to be configured repeatedly.
2. Matching Reagents
The following matching reagents are not included in this product but are required for detection:
Pre-excitation solution and excitation solution: Excite acridinium ester to catalyze the generation of photons, so that the luminescence intensity can be detected using a chemiluminescence immunoassay instrument.
Washing solution: Diluted for washing the reaction system.
Source: Provided by our company.
Note: In order to ensure the accuracy of the test results, the manufacturer of the matching reagents must not be changed arbitrarily during the test.
3. Calibrator Traceability
The calibrator can be traced to the enterprise's selected measurement procedure.
【Storage Conditions and Shelf Life】
The reagent is stable for 15 months at 2-8℃ away from light and should not be frozen.
After opening the liquid reagent, it is stable for 30 days at 2-8℃ away from light.
【Production Date】
See product label.
【Applicable Instruments】
Cosmay SMART500S, 6500 or Tuochuang Medical TC-300 fully automatic chemiluminescence immunoassay instrument
【Sample Requirements】
1. This kit is suitable for serum and whole blood samples.
2. After blood collection, serum should be separated immediately for analysis to avoid hemolysis. Testing should be completed within 24 hours at room temperature, stable for 3 days at 2-8℃, and stable for 30 days at -20℃. Avoid repeated freezing and thawing.
3. Frozen and refrigerated samples should be returned to room temperature and mixed thoroughly before use.
4. Avoid using samples with severe hemolysis or high concentrations of lipemia.
5. The sample volume should be no less than 200μL.
【Test Method】
Note: Please read the instructions carefully before use. Working conditions: Room temperature (15~35℃); Relative humidity ≤80%.
1. Pre-test preparation
1.1 Before the test, all reagents should be placed at room temperature, and the instrument should be preheated for at least 30 minutes;
1.2 According to the system operating instructions, perform the loading of reaction tubes (cups), the addition or replacement of matching reagents, waste liquid, waste tube cleaning, and liquid path filling operations.
2. Reagent and sample loading
2.1 Before reagent loading, place the reagents to be used on the mixing device for thorough mixing. Visually inspect the reagent solution components; they should be clear, free of foreign matter, precipitates, and flocculent matter. Magnetic microparticle reagents should be a uniform suspension without obvious aggregation;
2.2 According to the system operating instructions, scan the reagent barcodes to complete the loading of magnetic microparticle reagents and anti-reagents;
2.3 After mixing the calibrators and quality control samples, transfer them to the instrument's dedicated reaction cups and load them into the instrument's sample positions. After scanning, load the centrifuged serum samples directly into the instrument's sample positions.
3. Detection steps
For optimal detection performance, please follow the relevant instructions in this manual and operate according to the operating manual of the fully automated chemiluminescence analyzer.
Note: The detection reaction process and related parameters have been predefined in the instrument operating software.
4. Calibration curve and calibration
The calibration curve is obtained by scanning the main curve card to generate it directly, or by directly detecting six calibrator points to generate a fit, and the system also supports obtaining a working calibration curve using a two-point calibration method.
Note ①: Due to inter-instrument differences and system differences caused by operators, operating environments, and different accompanying general reagents, the six-point direct calibration method is recommended to obtain the working calibration curve.
Note ②: Due to reagent activity drift and changes in batches of general reagents, the calibrated working curve needs to be recalibrated after a certain period of use. Recalibration should be performed when the following situations occur:
l After 1 month (28 days) of using the same batch number of reagents;
l Using a new batch number of reagents, the whole box or pre-activation liquid and activation liquid;
l When the quality control values are outside the quality control range;
l After each maintenance of the analyzer.
5. Result output
The analyzer automatically calculates the CK-MB concentration of each sample using a working curve obtained by two-point calibration from the standard curve, and the results are expressed in ng/mL.
6. Quality control
Two levels of quality control samples should be determined daily at the same time as sample testing, treating the quality control samples as patient samples;
Note: It is recommended to use at least two levels of commercial quality control products. If the determination results are within the system's acceptable quality control range or within your specified range (determined by an appropriate internal laboratory quality control program), the test results are satisfactory; otherwise, it indicates that the test results are unreliable and a test report should not be issued.
【Reference Range】
Normal range of this kit: ≤4.99ng/mL.
Note: The CK-MB levels measured will vary depending on the region, individual, and method used for detection. Therefore, we recommend that each laboratory establish its own normal range. Diagnosis should not be made solely based on the CK-MB value obtained by this method; results should be analyzed in conjunction with other clinical data, including the patient's specific condition and treatment status.
【Interpretation of Test Results】
1. Due to methodological or antibody specificity reasons, testing the same sample using reagents from different manufacturers may yield different results. Therefore, results obtained using different reagents should not be directly compared to avoid erroneous medical interpretation.
2. Test results exceeding the measurement range of the kit are calculated results extrapolated from the calibrator curve. When reporting such results, please pay special attention. If an accurate value is desired, dilute the sample appropriately before testing; the maximum dilution factor is 10 times.
3. Quality control products can serve as a reference for the reliability of the results of the current experiment; their measured values should be within the allowable range specified on the quality control sheet for this batch of products. Test results should be judged comprehensively based on the reference range and other clinical factors and results. When the test result is close to the upper limit of the reference range, consider performing a confirmatory test on the sample.
【Limitations of the Test Method】
1. Severe hemolysis (hemoglobin ≥500mg/dL), lipemia (triglycerides ≥1500mg/dL), jaundice (bilirubin ≥20mg/dL), and contaminated samples may affect the test results and should be avoided.
2. The test results of this kit are for clinical reference only and cannot be used alone as the basis for confirming or excluding cases. To achieve diagnostic purposes, this test result should be used in conjunction with clinical examinations, medical history, and other examinations.
3. This product can be used for the determination of human serum samples; the reliability of determining CK-MB concentration in other body fluids has not been fully confirmed.
【Product Performance Indicators】
1. Reagent performance indicators
1.1 Accuracy: The recovery rate should be between 85%-115%.
1.2 Precision: Intra-batch precision ≤8%, inter-batch precision ≤10%.
1.3 Blank limit: Should not exceed 2 ng/mL.
1.4 Linearity: Linear range (2~500) ng/mL, correlation coefficient r≥0.9900.
1.5 Specificity: When detecting creatine kinase isoenzyme MM (CK-MM) at a concentration not higher than 35000 ng/mL, the measured result should not be higher than 2 ng/mL; when detecting creatine kinase isoenzyme BB (CK-BB) at a concentration not higher than 120 ng/mL, the measured result should not be higher than 2 ng/mL.
2. Calibrator Performance Indicators
2.1 Accuracy: Recovery rate should be between 85%-115%.
2.2 Homogeneity: Inter-bottle difference (CV) ≤8%.
3. Quality Control Product Performance Indicators
3.1 Expected results: Test values should be within ±15% of the target value of the quality control product.
3.2 Homogeneity: Inter-bottle difference (CV) ≤8%.
【Precautions】
1. This reagent is for in vitro diagnostic use only. Reagents from different batches are prohibited from being used interchangeably. Do not use expired reagents.
2. Some components of this kit contain bovine-derived materials, but these raw materials are not from countries or regions with bovine spongiform encephalopathy (BSE) outbreaks, and their production is not carried out in countries or regions with BSE outbreaks. If it is bovine serum, it will be filtered and sterilized after purchase before being used in kit production. Therefore, the kit does not pose a risk of transmitting BSE; some components of the kit contain porcine-derived materials, but these raw materials are not from countries or regions with foot-and-mouth disease outbreaks, and their production is not carried out in countries or regions with foot-and-mouth disease outbreaks. If it is porcine serum, it will be filtered and sterilized after purchase before being used in kit production. Therefore, the kit does not pose a risk of transmitting foot-and-mouth disease; this reagent does not contain human serum, but should still be considered a potential biohazard.
3. This reagent contains preservatives. Avoid contact with skin. If skin accidentally comes into contact with the reagent, rinse thoroughly with plenty of water.
4. All reagents should be stored at 2~8℃, avoid freezing at -20℃, and equilibrate to room temperature before use.
5. The magnetic microparticle reagent should be thoroughly mixed before use, and avoid strong shaking.
【Explanation of Labels】
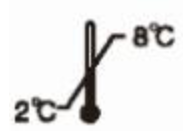 Store at 2-8℃
Store at 2-8℃  Avoid direct sunlight
Avoid direct sunlight  Place upright
Place upright
 In vitro diagnostic reagent
In vitro diagnostic reagent  Production date
Production date 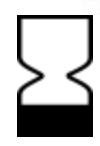 Expiration date
Expiration date
 Product batch number
Product batch number 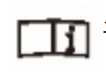 See instructions for use
See instructions for use
【References】
1. Panteghini M, Pagani F. AACC creatine kinase MB (CK-MB) standardization material used as manufacturer's working calibrator is unable to harmonize CK-MB results between two commercial immunoassays. Clinical Chemistry, 2004, 50(9):1711.
2. Members C, Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin M.D, et al. Acc/aha guideline update for the management of patients with unstable angina and non-st-segment elevation myocardial infarction 2002: summary article. Circulation. 2002, 106(14):1893-900.
3. Wu All, Feng Yue Jm, Contob JH, et al. Comparison of myoglobm Creatine Kinase-MB and cardiac troponin I for diagnoais of acute myocardial iIIfarefion. Ann ClilI Lab Sci. 1996(26) : 291-292.
4. Sun Chunxia. Clinical value of creatine kinase isoenzyme in the diagnosis of acute myocardial infarction. Chinese Contemporary Medicine, 2014, 21(27):106-107.
【Basic Information】
Registrant/Manufacturer: Zhejiang Tuochuang Medical Technology Co., Ltd
Address: No. 1 Building, Qianren Plan Industrial Park, No. 78, Zhancheng Avenue, Zhuji City, Zhejiang Province
Production address: No. 1 Building, Qianren Plan Industrial Park, No. 78, Zhancheng Avenue, Zhuji City, Zhejiang Province
After-sales service unit: Zhejiang Tuochuang Medical Technology Co., Ltd
Production license number:
Contact number: 0575-87178692
Fax:
Post code: 311800
Website: www.zjtcyl.com
【Medical Device Registration Certificate Number/Product Technical Requirements Number】
【Date of Approval and Revision of Instructions for Use】
Approval date:
Revision date:
Previous Page
Next Page
Related Downloads
Related Products
Contact Us
Adhering to the user-centric approach, while continuously improving service standards and capabilities, we will remain true to our original aspiration, strive for excellence, and wholeheartedly serve our customers!
Contact Number
Company Address
No. 1, Building 1, No. 78, Zhancheng Avenue, Taozhu Street, Zhuji City, Zhejiang Province

Scan to view on mobile phone
Copyright © 2025 Zhejiang Tuochuang Medical Technology Co., Ltd Powered by 300.cn
