Prolactin (PRL)
The Prolactin (PRL) Assay Kit (magnetic microparticle chemiluminescence method) (hereinafter referred to as this kit) is used for in vitro quantitative detection of prolactin (PRL) in human serum. It is used to assist in the diagnosis of male infertility, female infertility, and pituitary dysfunction.
Keywords:
Sex hormone
Category:
Sex hormone
Brochure Download:

Tel:
Prolactin (PRL) Assay Kit
Instructions for Use (Magnetic Microparticle Chemiluminescence Method)
Instruction of Prolactin (PRL) Detection Kit (Magnetic Solid Phase Chemiluminescent Immunoassay)
【Product Name】
Generic Name: Prolactin (PRL) Assay Kit (Magnetic Microparticle Chemiluminescence Method)
English Name: Prolactin (PRL) Detection Kit (Magnetic Solid Phase Chemiluminescent Immunoassay)
【Packaging Specifications】
50 tests/box, 100 tests/box, 200 tests/box.
Calibrator: 6 × 1 bottle (optional), 2 × 1 bottle (optional)
Quality Control: 2 × 1 bottle (optional)
【Intended Use】
The Prolactin (PRL) Assay Kit (Magnetic Microparticle Chemiluminescence Method) (hereinafter referred to as this kit) is used for the in-vitro quantitative detection of prolactin (PRL) in human serum. It is used as an aid in the diagnosis of male infertility, female infertility, and pituitary dysfunction.
Prolactin (Prolactin, PRL) is a protein hormone secreted by acidophilic cells in the anterior pituitary gland. It is a single-chain polypeptide composed of 199 amino acids, containing three disulfide bonds, with a chemical structure similar to growth hormone, belonging to the growth hormone family, and its relative molecular mass is 23,000 daltons. Various PRL molecules of different sizes also exist in the blood. PRL's main functions include promoting mammary gland development and growth, stimulating and maintaining lactation, and stimulating the generation of follicle LH receptors. PRL promotes mammary gland development and growth, causing and maintaining lactation. During pregnancy, PRL, human chorionic somatomammotropin, estrogen, and progesterone further develop mammary tissue. After delivery, the concentration of estrogen and progesterone in the blood decreases, and PRL initiates and maintains lactation. Clinically, in non-lactating women, high PRL levels lead to galactorrhea and amenorrhea; patients generally have anovulation and low estrogen levels. In men, in the presence of testosterone, PRL promotes the growth of the prostate and seminal vesicles and can enhance the effect of luteinizing hormone on interstitial cells, increasing testosterone synthesis. Clinically, in addition to diseases, there are many factors that affect PRL levels. Factors that cause PRL to rise include: pregnancy, breast stimulation, use of estrogen, androgens, mental stress, chest wall trauma, surgery, and herpes zoster, etc. Factors that reduce PRL concentration include the use of levodopa and bromocriptine. Common laboratory diagnostic methods in clinical practice include immunochromatography and chemiluminescence.
【Testing Principle】
This kit uses a direct sandwich method: biotin-labeled PRL antibody, acridinium ester (AE)-labeled PRL antibody, and PRL in the sample, calibrator, or quality control combine to form a "sandwich" complex. Subsequently, streptavidin-coated magnetic microparticles are added. Through the specific binding of streptavidin and biotin, the antigen-antibody complex is connected to the magnetic particles. Under the action of an external magnetic field, the complex formed by the immunoreaction is separated from other unbound substances. After washing the complex, a pre-excitation solution and an excitation solution are added. The acridinium ester forms an unstable excited intermediate under the action of the pre-excitation solution and the excitation solution. When the excited intermediate returns to the ground state, it emits photons, forming a chemiluminescent reaction, which can be detected using a chemiluminescence instrument. Within the detection range, the chemiluminescence intensity is proportional to the PRL content in the sample. The PRL concentration in the sample can be calculated using a four-parameter Logistic equation.
【Main Components】
1. Product Composition
PRL magnetic microparticle reagent: 1 bottle, streptavidin-coated magnetic microparticles;
PRL antibody reagent 1: 1 bottle, biotin-labeled PRL antibody conjugate;
PRL antibody reagent 2: 1 bottle, acridinium ester (AE)-labeled PRL antibody conjugate;
Calibrator (lyophilized powder): 6 bottles or 2 bottles, containing BSA buffer with different amounts of PRL antigen added. The calibrator value is obtained by scanning the calibrator QR code. After dissolving at room temperature, perform the test;
Quality control (lyophilized powder): 1 bottle each of high and low points (optional), containing BSA buffer with different amounts of PRL antigen added. The target range of the quality control is obtained by scanning the quality control QR code. After dissolving at room temperature, perform the test.
Note ①: Reagents from different batches of kits are prohibited from being used interchangeably.
Note ②: Calibrators can be optionally configured with six calibration points or two calibration points. If calibrators and quality controls have already been provided to the user, the kit does not need to be re-configured.
2. 配套试剂
The following reagents are not included in this product but are required for the test:
Pre-excitation solution and excitation solution: Excite acridinium ester to catalyze the production of photons, so that the chemiluminescence intensity can be detected using a chemiluminescence immunoassay instrument.
Washing solution: Diluted for washing the reaction system.
Source: Provided by our company.
Note: To ensure the accuracy of the test results, the manufacturer of the accompanying reagents should not be changed arbitrarily during the test.
3. Calibrator Traceability
The calibrator is traceable to the "National Standard Substance for Prolactin (PRL) Immunoassay" provided by the China National Institute for Food and Drug Control.
【Storage Conditions and Shelf Life】
The reagents are stable for 15 months when stored at 2-8℃, protected from light, and should not be frozen.
After opening, liquid reagents are stable for 30 days at 2-8℃, protected from light.
【Production Date】
See product label.
Applicable Instruments
Cosmed SMART500S, 6500 or Tuochuang Medical TC-300 fully automated chemiluminescence immunoassay analyzer
Sample Requirements
1. This kit is suitable for serum samples.
2. After blood collection, serum should be separated immediately for analysis to avoid hemolysis. Testing must be completed within 24 hours at room temperature; it is stable for 3 days at 2-8℃ and 30 days at -20℃. Avoid repeated freezing and thawing.
3. Frozen or refrigerated samples should be returned to room temperature and mixed thoroughly before use.
4. Avoid using samples with severe hemolysis or high levels of lipemia.
5. The sample volume should be no less than 200μL.
Testing Method
Note: Please read the instructions carefully before use. Working conditions: Room temperature (15~35℃); Relative humidity ≤80%.
1. Pre-test preparation
1.1 Before the test, all reagents should be placed at room temperature, and the instrument should be preheated for at least 30 minutes;
1.2 According to the system operating instructions, perform the loading of reaction tubes (cups), the addition or replacement of matching reagents, waste liquid, waste tube cleaning, and liquid path filling operations.
2. Reagent and sample loading
2.1 Before reagent loading, place the reagents to be used on a mixing device and mix thoroughly. Visually inspect the reagent solution components; they should be clear, free of foreign matter, precipitates, and flocculent matter. Magnetic microparticle reagents should be a uniform suspension without obvious aggregation;
2.2 According to the system operating instructions, scan the reagent barcodes to complete the loading of magnetic microparticle reagents and anti-reagents;
2.3 After mixing the calibrators and quality control samples, transfer them to the instrument's dedicated reaction cups and load them into the instrument's sample positions. After scanning, load the centrifuged serum samples directly into the instrument's sample positions.
3. Detection steps
For optimal detection performance, please follow the relevant guidance in this instruction manual and operate according to the fully automated chemiluminescence analyzer operating manual.
Note: The detection reaction process and related parameters have been predefined in the instrument operating software.
4. Calibration curve and calibration
The calibration curve is obtained by scanning the main curve card to generate it directly, or by directly detecting six calibrator points to generate a fit, and the system supports obtaining a working calibration curve using a two-point calibration method.
Note ①: Due to inter-instrument differences and system differences caused by different operators, operating environments, and matching general reagents, the six-point direct calibration method is recommended to obtain the working calibration curve.
Note ②: Due to reagent activity drift and changes in general reagent batches, the calibrated working curve needs to be recalibrated after a certain period of use. Recalibration should be performed under the following circumstances:
l After 1 month (28 days) of using the same batch number of reagents;
l When using a new batch of reagents, the whole box or pre-activation liquid and activation liquid;
l When the quality control values are outside the quality control range;
l After each maintenance of the measuring instrument.
5. Result output
The measuring instrument automatically calculates the PRL concentration of each sample using a working curve obtained by two-point calibration from the standard curve, and the results are expressed in μIU/mL.
6. Quality control
6.1 Two levels of quality control samples should be measured daily along with sample testing, treating the quality control samples as patient samples;
Note: It is recommended to use at least two levels of commercial quality control products. If the measured results are within the system's acceptable quality control range or within your specified range (determined by an appropriate internal laboratory quality control program), the test results are satisfactory; otherwise, it indicates that the test results are unreliable and a test report should not be issued.
Reference Range
|
Gender |
Reference Range (μIU/mL) |
|
Male |
86-324 |
|
Female (Non-pregnant) |
102-496 |
Note: The PRL levels measured may vary depending on the region, individual, and method used. Therefore, we recommend that each laboratory establish its own normal value range. A diagnosis should not be made based solely on the PRL value obtained by this method; the results should be analyzed in conjunction with other clinical data, including the patient's specific condition and treatment status.
Interpretation of Test Results
1. Due to methodological or antibody specificity reasons, testing the same sample using reagents from different manufacturers may yield different results. Therefore, results obtained using different reagents should not be directly compared to avoid erroneous medical interpretation.
2. Test results exceeding the measurement range of the kit are calculated results extrapolated from the calibrator curve. When reporting such results, please pay special attention. If an accurate value is desired, dilute the sample appropriately before testing; the maximum dilution factor is 100 times.
3. Quality control products can serve as a reference for the reliability of the results of the current experiment; their measured values should be within the allowable range specified on the quality control sheet for this batch of products. Test results should be judged comprehensively based on the reference range and other clinical factors and results. When the test result is close to the upper limit of the reference range, consider performing a confirmatory test on the sample.
Limitations of the Test Method
1. Severe hemolysis (hemoglobin ≥500mg/dL), lipemia (triglycerides ≥1500mg/dL), jaundice (bilirubin ≥20mg/dL), and contaminated samples may affect the test results and should be avoided.
2. The test results of this kit are for clinical reference only and cannot be used alone as the basis for confirming or excluding cases. To achieve diagnostic purposes, this test result should be used in conjunction with clinical examinations, medical history, and other examinations.
3. This product can be used for the determination of human serum samples. The reliability of determining PRL concentration in other body fluid samples has not been fully confirmed.
Product Performance Indicators
1. Reagent Performance Indicators
1.1 Accuracy: The ratio of the measured value to the labeled value of the calibrators in the kit should be between 0.900 and 1.100.
1.2 Precision: Intra-batch precision ≤8%, inter-batch precision ≤10%.
1.3 Blank limit: Should not exceed 5.0 μIU/mL.
1.4 Linearity: In the range of (5.0~5000.0) μIU/mL, the correlation coefficient r should be ≥0.9900.
1.5 Specificity: When measuring 200 IU/L of FSH, the measured result should not exceed 5.0 μIU/mL; when measuring 200 ng/mL of human growth hormone (hGH), the measured result should not exceed 5.0 μIU/mL.
2. Calibrator Performance Indicators
2.1 Accuracy: The ratio of the measured value to the labeled value of the calibrators in the kit should be between 0.900 and 1.100.
2.2 Homogeneity: Inter-bottle difference (CV) ≤8%.
3. Quality Control Product Performance Indicators
3.1 Expected results: The test value should be within ±15% of the target value of the quality control product.
3.2 Homogeneity: Inter-bottle difference (CV) ≤8%.
【Precautions】
1. This reagent is for in vitro diagnostic use only. Reagents from different batches are prohibited from being used interchangeably. Do not use expired reagents.
2. Some components of this kit contain bovine-derived materials, but these raw materials are not from countries or regions with bovine spongiform encephalopathy (BSE) epidemics, and their production is not carried out in countries or regions with BSE epidemics. If it is bovine serum, it will be filtered and sterilized after purchase before being used in kit production. Therefore, the kit does not pose a risk of transmitting BSE. Some components of this kit contain porcine-derived materials, but these raw materials are not from countries or regions with foot-and-mouth disease epidemics, and their production is not carried out in countries or regions with foot-and-mouth disease epidemics. If it is porcine serum, it will be filtered and sterilized after purchase before being used in kit production. Therefore, the kit does not pose a risk of transmitting foot-and-mouth disease. This reagent does not contain human serum, but it should still be considered a potential biohazard.
3. This reagent contains preservatives. Avoid contact with skin. If skin accidentally comes into contact with the reagent, rinse thoroughly with plenty of water.
4. All reagents should be stored at 2~8℃, avoid freezing at -20℃, and equilibrate to room temperature before use.
5. The magnetic microparticle reagent should be thoroughly mixed before use, and strong shaking should be avoided.
【Explanation of Labels】
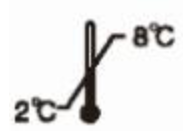 Store at 2-8℃
Store at 2-8℃  Avoid direct sunlight
Avoid direct sunlight  Store upright
Store upright
 In vitro diagnostic reagent
In vitro diagnostic reagent  Production date
Production date 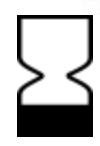 Expiration date
Expiration date
 Product batch number
Product batch number 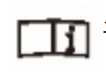 See instructions for details
See instructions for details
【References】
1. Clevenger CV, Fu rth PA, Hankinson S E, et al. The role of prolactin in mammary carcinoma, Endocr. Rev, 2003 , 24(1):1-27.
2. Qiu Daiyu. Changes in serum prolactin levels in normal pregnant women. China Practical Medicine, 2012, 07(15):110-111.
3. Frantz AG. Prolactin. N Engl J Med 1978;298:201-207.
4. Jiang Lihui, Gui Suiqi, Li Kunming, et al. Effect of traditional Chinese medicine on serum PRL in a bromocriptine-induced abortion model. Reproduction and Contraception, 2002, 22(4):199-202.
5. Gu Limei. Correlation between serum prolactin levels and dementia in Parkinson's disease. 2007,24(3): 291-293.
【Basic Information】
Registered person/manufacturer: Zhejiang Tuochuang Medical Technology Co., Ltd
Address: No. 1 Building, Qianren Plan Industrial Park, No. 78, Zhancheng Avenue, Zhuji City, Zhejiang Province
Production address: No. 1 Building, Qianren Plan Industrial Park, No. 78, Zhancheng Avenue, Zhuji City, Zhejiang Province
After-sales service unit: Zhejiang Tuochuang Medical Technology Co., Ltd
Production license number:
Contact number: 0575-87178692
Fax:
Post code: 311800
Website: www.zjtcyl.com
【Medical Device Registration Certificate Number/Product Technical Requirements Number】
【Date of Approval and Revision of Instructions for Use】
Approval date:
Revision date:
Previous Page
Next Page
Previous Page
Next Page
Related Downloads
Related Products
Contact Us
Adhering to the user-centric approach, while continuously improving service standards and capabilities, we will remain true to our original aspiration, strive for excellence, and wholeheartedly serve our customers!
Contact Number
Company Address
No. 1, Building 1, No. 78, Zhancheng Avenue, Taozhu Street, Zhuji City, Zhejiang Province

Scan to view on mobile phone
Copyright © 2025 Zhejiang Tuochuang Medical Technology Co., Ltd Powered by 300.cn
